Lead Program: Egafusp
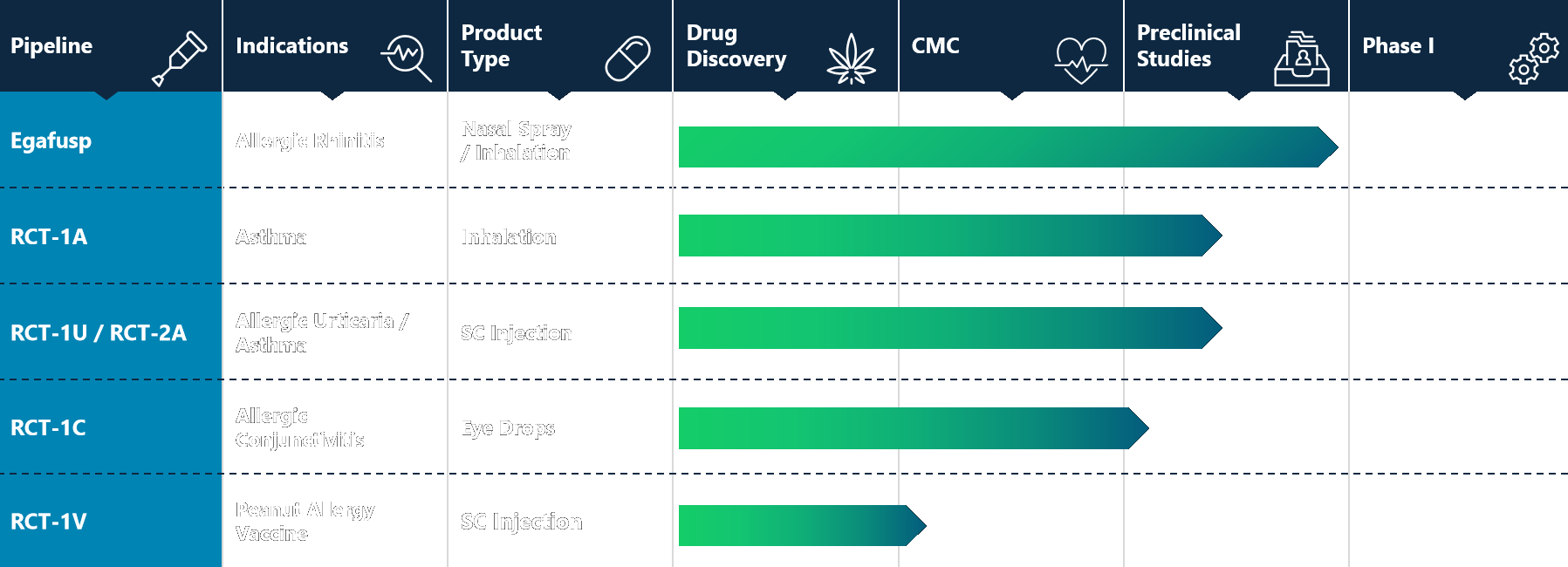
Pipeline Overview
RiverCreek Therapeutics is developing a fusion-protein therapeutic platform based on a unique mechanism of action that selectively modulates IgE-mediated allergic signaling. By targeting a central upstream pathway in allergic inflammation, this approach is designed to support broad applicability across allergic diseases.
The Company’s lead program, Egafusp, is being developed as a nasal-spray biologic for the treatment of allergic rhinitis. The same underlying molecular platform provides a foundation for future pipeline expansion across additional allergic indications.