MILESTONE
Advancing Inhaled Biologic Therapy
A major milestone in the development of Egafusp has been the successful formulation of the molecule as a stable dry-powder protein.
This innovation enables:
Intranasal delivery
Intranasal delivery for allergic rhinitis
Inhalation therapy
Inhalation therapy for allergic asthma and other respiratory allergic diseases
Why Egafusp Is Different ?
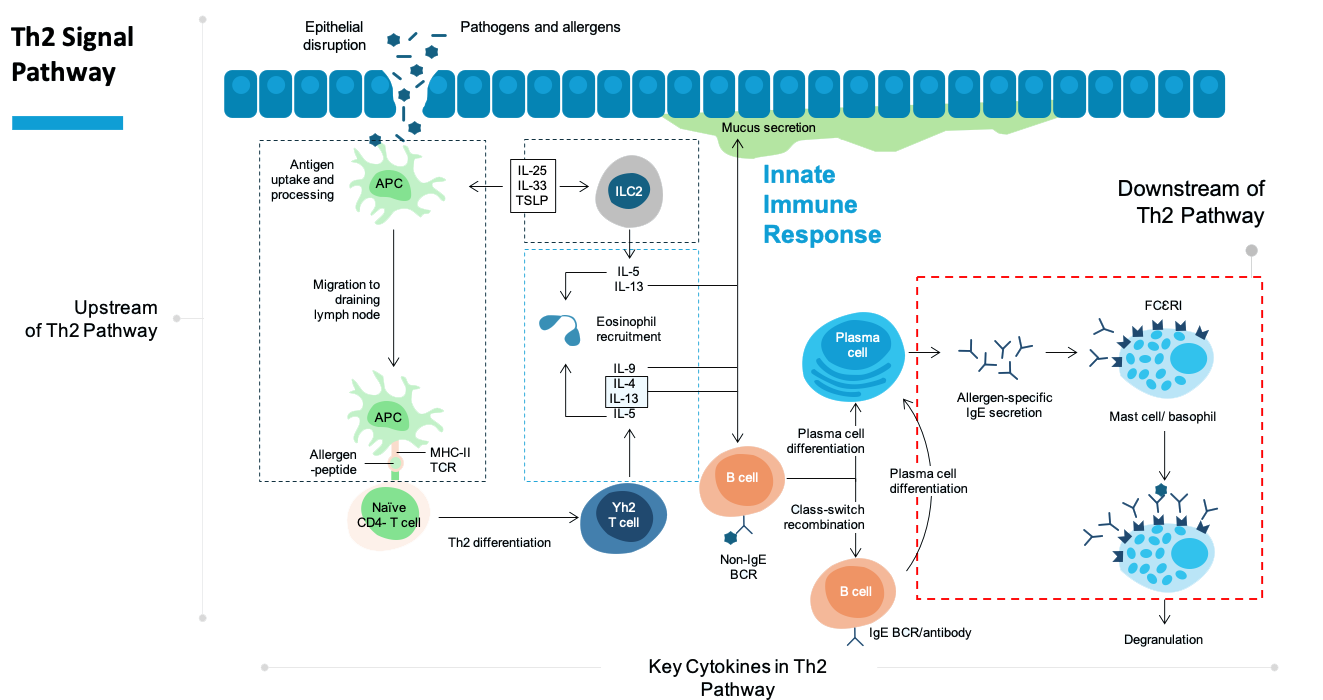
Biologic medicines have become increasingly important for treating allergic diseases, especially for patients whose symptoms are not well controlled by standard treatments like antihistamines, steroid sprays, or other allergy medications. These biologics can be very effective—but today, they also come with major limitations.
Most existing biologic allergy treatments must be given by injection, often in a doctor’s office. They usually need to be stored in a refrigerator and cannot be used as nasal sprays or inhalers. This makes treatment inconvenient, limits flexibility, and can be burdensome for patients who need long-term care.
Egafusp is designed to change that.
Egafusp is a biologic therapy formulated as a stable dry powder that can be delivered directly to the nose or lungs—right where allergic inflammation begins. It is easy for patients to self-administer, without injections or clinic visits. Unlike traditional biologics, Egafusp can be stored at room temperature for over a year, allowing patients to carry and use their medication without worrying about refrigeration or loss of activity.
By combining the effectiveness of biologic therapy with the convenience of nasal and inhaled delivery, Egafusp aims to remove key barriers to treatment and offer patients a safer, more practical, and more flexible option for managing allergic diseases.